Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
斉藤 淳一; Monbernier, M.*
Surfaces and Interfaces (Internet), 41, p.103248_1 - 103248_8, 2023/10
被引用回数:0 パーセンタイル:0.01(Chemistry, Physical)Contact angle is an indicator of the wettability between liquid Na and pure metals. This has been evaluated using the atomic interactions obtained from the calculations of the electronic structure of the interface. This study aims to investigate the applicability of the atomic interactions of the interface to the alloys. An interface model between Cu and Na with an alloying element was constructed, and the electronic states of the interface were calculated by the molecular orbital calculation. The bond order, which indicates the strength of the covalent bonding at the interface, and ionicity, which indicates the amount of charge transfer, were obtained as theoretical parameters from the calculation. The contact angles between the Cu or Cu alloys and liquid Na were measured using a droplet of liquid Na at 423 K in a high-purity Ar atmosphere. The contact angles of the Cu alloys were evaluated using these theoretical parameters. As a result, a correlation was obtained between the ratio of the bond order between the substrate metal atoms to the bond order between the Na atom and the substrate metal atoms and the contact angle, which is consistent with previous studies. Furthermore, for the first time, the correlation between the ionicity or difference in the ionicity and contact angle was clarified. The difference in ionicity is the difference between the ionicity of Na atoms and that of the alloying element, indicating the strength of the ionic bonding. It was suggested that Cu and Cu alloys should consider covalent and ionic bonding when evaluating wettability, because Cu has an intermediate electronic state between transition and nontransition metals. Further, it became clear that the evaluation of the contact angle using the atomic interactions at the interface are applicable not only to pure metals but also to alloys.
小川 徹; J.K.Gibson*; R.G.Haire*; M.M.Gensini*; 赤堀 光雄
Journal of Nuclear Materials, 223, p.67 - 71, 1995/00
被引用回数:15 パーセンタイル:79.37(Materials Science, Multidisciplinary)アクチニド金属f状態と遷移金属のd状態の混合は金属と組成の双方に依存する。Zr-U系には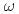 相固溶体と見なすことのできる中間相
相固溶体と見なすことのできる中間相 が存在する。すなわちU添加により
が存在する。すなわちU添加により 相が
相が 相(hcp)に対して安定化される。このことはUの原子価殻とZrのd殻とが良く混合した結果、Zr-d帯の占有率が上昇したことを意味すると考えられる。Zr-Np系にも
相(hcp)に対して安定化される。このことはUの原子価殻とZrのd殻とが良く混合した結果、Zr-d帯の占有率が上昇したことを意味すると考えられる。Zr-Np系にも 類似相が存在する。しかし、
類似相が存在する。しかし、 -Zrと
-Zrと -Uとがbcc金属として良く相互固溶するのに対し、
-Uとがbcc金属として良く相互固溶するのに対し、 -Zrと
-Zrと -Npとの相互溶解度は限られるようである。Np-Zr系の熱力学的解析の結果、Zr-Np間の相互作用がZr-U間のそれと同程度であれば、bcc領域の溶解度差曲線が固相線と交わることが明らかになった。これらの議論の基礎となるU-4d遷移金属合金化の系統的挙動についても論じた。
-Npとの相互溶解度は限られるようである。Np-Zr系の熱力学的解析の結果、Zr-Np間の相互作用がZr-U間のそれと同程度であれば、bcc領域の溶解度差曲線が固相線と交わることが明らかになった。これらの議論の基礎となるU-4d遷移金属合金化の系統的挙動についても論じた。
 and Li
and Li MoO
MoO
佐々木 貞吉; 木内 清
Chemical Physics Letters, 84(2), p.356 - 360, 1981/00
被引用回数:18 パーセンタイル:58.06(Chemistry, Physical)MoO およびMoO
およびMoO

 イオンに対してそれぞれ〔Mo
イオンに対してそれぞれ〔Mo O
O
 〕
〕

 、〔MoO
、〔MoO 〕
〕
 クラスターを仮定し、電子状態とレベル構造をDV-X
クラスターを仮定し、電子状態とレベル構造をDV-X 分子軌道法で計算した。えられた結果を検討し、MoO
分子軌道法で計算した。えられた結果を検討し、MoO よりもMoO
よりもMoO

 イオンのMo-Oがより共有結合性を帯びるという知見をえた。このことはMo3dのXPSスペクトル測定からも確かめられた。また、価電子帯レベル構造は実測スペクトルと良好な対応を示すことが判明した。
イオンのMo-Oがより共有結合性を帯びるという知見をえた。このことはMo3dのXPSスペクトル測定からも確かめられた。また、価電子帯レベル構造は実測スペクトルと良好な対応を示すことが判明した。